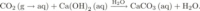Moving-boundary-modelling of concrete carbonation
| Working Group: | Former WG Modelling and PDEs |
| Leadership: | Prof. Dr. Michael Böhm (E-Mail: mbohm@math.uni-bremen.de ) |
| Processor: | Prof. Dr. Adrian Muntean |
| Funding: | DFG SPP 1122 |
| Project partner: |
Labor für Baustofftechnologie, Hochschule Bremen Baustoffinstitut TU Dresden |
| Time period: | 01.07.2001 - 30.06.2006 |
 Reinforced concrete is a widely used construction material. It is not only cheap but, in contrast to other materials such as timber or steel, very durable. However, in the last decades it has become clear that the durability of reinforced concrete is strongly affected by the corrosion of the steel bars in its interior. Usually, the steel bars are protected by a thin oxide layer at their surface which is formed because of the highly alkaline environment. The high pH in concrete (about 13) is mostly due to the calcium hydroxide produced during the curing period. Since concrete is a porous material, gaseous CO2 diffuses into its interior under natural environmental conditions where it reacts with available Ca(OH)2 to produce CaCO3 (and H2O). While the carbonation does not present any immediate danger for the set cement (the strength can even increase), it lowers the pH in the concrete and therefore allows the corrosion of the reinforcing steel bars. This phenomenon is considered as one of the major processes inducing corrosion in concrete and leads to an enormous loss in the range of the upper hundreds of millions Euro in Germany annually. It is therefore of great importance to have simple (i.e. easily computable) models which predict the carbonation penetration accurately.
Reinforced concrete is a widely used construction material. It is not only cheap but, in contrast to other materials such as timber or steel, very durable. However, in the last decades it has become clear that the durability of reinforced concrete is strongly affected by the corrosion of the steel bars in its interior. Usually, the steel bars are protected by a thin oxide layer at their surface which is formed because of the highly alkaline environment. The high pH in concrete (about 13) is mostly due to the calcium hydroxide produced during the curing period. Since concrete is a porous material, gaseous CO2 diffuses into its interior under natural environmental conditions where it reacts with available Ca(OH)2 to produce CaCO3 (and H2O). While the carbonation does not present any immediate danger for the set cement (the strength can even increase), it lowers the pH in the concrete and therefore allows the corrosion of the reinforcing steel bars. This phenomenon is considered as one of the major processes inducing corrosion in concrete and leads to an enormous loss in the range of the upper hundreds of millions Euro in Germany annually. It is therefore of great importance to have simple (i.e. easily computable) models which predict the carbonation penetration accurately.
To predict the advancement of carbonation fronts in concrete, systems of partial and ordinary differential equations involving the exchange of constituents across pore-water and pore-air as well as pore-water and solid-matrix interfaces as well as chemical reactions in the water-filled part of the pores are employed. We are considering the carbonation reaction in the form We focus on the following aspects: